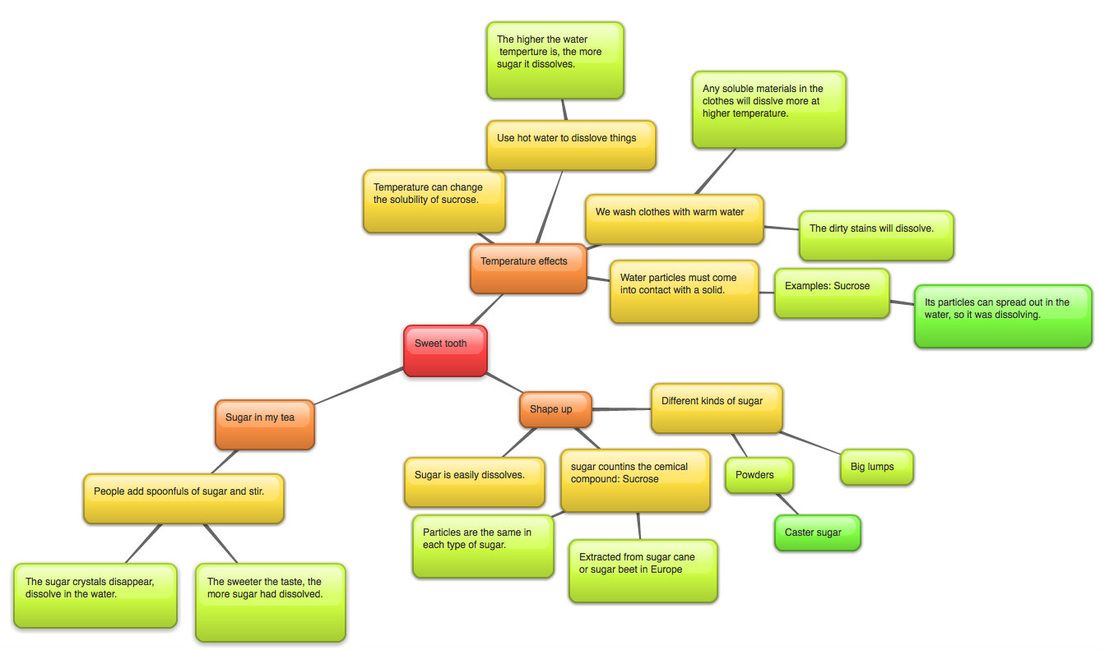
Question 1: How does the temperature of the solvent affect the amount of solute that is dissolved?
When the solvent's temperature is high, its particles get active and they stay away from each other. This make place for the solute particles, then they can come together more easily. Also, the active particles from the solvent would contact with the solute particles and there are more energy for them to pull the solute particles closer,so the solute has been dissolved.
Question 2: Why do you think the temperature affects the amount of solute dissolved?
The temperature affects the amount of solute dissolved because I think the higher the temperature, solute get more soluble, so they can be dissolved quicker. As the more solute dissolved in the solvent, the solvent becomes more concentrate (that means the more H2O it has). The more concentrate of the solvent, the more solute it can dissolve.
When the solvent's temperature is high, its particles get active and they stay away from each other. This make place for the solute particles, then they can come together more easily. Also, the active particles from the solvent would contact with the solute particles and there are more energy for them to pull the solute particles closer,so the solute has been dissolved.
Question 2: Why do you think the temperature affects the amount of solute dissolved?
The temperature affects the amount of solute dissolved because I think the higher the temperature, solute get more soluble, so they can be dissolved quicker. As the more solute dissolved in the solvent, the solvent becomes more concentrate (that means the more H2O it has). The more concentrate of the solvent, the more solute it can dissolve.
 RSS Feed
RSS Feed