INTRODUCTION:
Mixtures are substances that held together by physical forces, and their individual structures and properties remain the same. They are easily separated by physical means.Mixtures are heterogenous. Solutions are also mixtures but substances are held together by chemical reaction and they are homogenous. The difference between a heterogenous and a homogenous is that heterogenous are mixtures that are not evenly mixed, but homogenous are. Solvents and solute are included in solutions because solvents dissolved solutes, so they became a solution. In our experiments, it's always connected to evaporation,condensation and distillation.
Evaporation is a process of liquid turning to gas because of heat. Condensation is the opposite of it. It's a process of gas turning to liquid because of cold. And distillation is the mixture of evaporation and condensation. That means when we are doing distillation, we are doing evaporation and condensation. The process of distillation is
Mixtures are substances that held together by physical forces, and their individual structures and properties remain the same. They are easily separated by physical means.Mixtures are heterogenous. Solutions are also mixtures but substances are held together by chemical reaction and they are homogenous. The difference between a heterogenous and a homogenous is that heterogenous are mixtures that are not evenly mixed, but homogenous are. Solvents and solute are included in solutions because solvents dissolved solutes, so they became a solution. In our experiments, it's always connected to evaporation,condensation and distillation.
Evaporation is a process of liquid turning to gas because of heat. Condensation is the opposite of it. It's a process of gas turning to liquid because of cold. And distillation is the mixture of evaporation and condensation. That means when we are doing distillation, we are doing evaporation and condensation. The process of distillation is
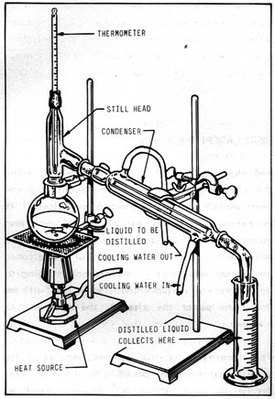
first, we heat up the liquid, when it reaches it's boiling point, it will start evaporate. The gas then go into the liebig condenser where the gas cool down and become liquid again. The pure liquid then goes into a testing tube.This process separate substances that had been dissolved into the liquid.
OBJECTIVE:
We were doing this experiment because we want to know what is distillation and how does it work. Also we can know what is the difference between condensation, evaporation and distillation, and what we can get when things were distillate.
HYPOTHESIS:
After distilling the brine solution, the pure salt and water would be separate.
MATERIALS:
Retort stands and clamps (2)
Boiling tube (1)
Rubber Bung (1)
Glass tube (1)
Conical flask (1)
Goggles (2)
wooden stick (1)
Lighter (1)
Chemical:
Lead Nitrate
We were doing this experiment because we want to know what is distillation and how does it work. Also we can know what is the difference between condensation, evaporation and distillation, and what we can get when things were distillate.
HYPOTHESIS:
After distilling the brine solution, the pure salt and water would be separate.
MATERIALS:
Retort stands and clamps (2)
Boiling tube (1)
Rubber Bung (1)
Glass tube (1)
Conical flask (1)
Goggles (2)
wooden stick (1)
Lighter (1)
Chemical:
Lead Nitrate
PROCEDURE:
1. Set up. Use the retord stands and clamps to clamp the conical flask (with brine solution in it) and testing tube.Put the conical flask on top of the bunsen burner set.
2. Connect the tube from the bunsen burner to the gas tap. Light up the wooden stick and put it on top of the bunsen burner, then open the gas. After that open the air hole.
3. Wait until the brine solution was boiled and the water turned to gas and cool down and turn to liquid again. Then the salt and the water were separate.
4. Use dropper to put a few drops of lead nitrate into the water in the testing tube. Then see if the water change colour or not. If the water change colour (white) that means the water is not really pure, if not, that means the water is pure.
1. Set up. Use the retord stands and clamps to clamp the conical flask (with brine solution in it) and testing tube.Put the conical flask on top of the bunsen burner set.
2. Connect the tube from the bunsen burner to the gas tap. Light up the wooden stick and put it on top of the bunsen burner, then open the gas. After that open the air hole.
3. Wait until the brine solution was boiled and the water turned to gas and cool down and turn to liquid again. Then the salt and the water were separate.
4. Use dropper to put a few drops of lead nitrate into the water in the testing tube. Then see if the water change colour or not. If the water change colour (white) that means the water is not really pure, if not, that means the water is pure.
OBSERVATION/RESULTS:
After turn on the bunsen burner for a few minutes, the brine solution started boiling. Then the water in the brine started evaporate and went into the glass tube. The glass tube cool down the gas and it turn ti liquid again. After some times, the water form the brine were all evaporated and it left with pure salt, and the water were in the testing tube.
DISCUSSION AND CONCLUSION:
The brine solution was supposed to boiled and the final result would be pure salt and water. Overall my distillation set up was successful because I did get pure salt and water at the end but the many steam that comes out from the conical flask escape, so that I did not get much water at the end. If I can do this experiment again, I will put the other end of the glass tube into a rubber bung and put the rubber bung into the test tube, then the steam can't escape. But I do it this way the glass tube might get hot quickly, then the steam can't turns into water. We add lead nitrate into the water at the end of the experiment because we want to know is the water pure or not.
I leant many things in this experiment: We need to be cooperate with our partners then we could have a quicker and better result. How distillation works, and what is the result (the solvent and solute would separate)... I really enjoy doing this experiment because this is my first time doing distillation experiment and using a retord stands and clamps.
 RSS Feed
RSS Feed